COVID-19 Antibody Responses in Cystic Fibrosis (CAR-CF)
Aim
The virus SARS-CoV-2 has caused a global pandemic of Covid-19. Clinical trial networks in Canada, Europe and the USA have worked together to set up a study called CAR-CF. This study will collect blood samples from thousands of people with CF to see whether the person had a previous SARS-CoV-2 infection. The study will also explore the level of antibodies to SARS-CoV-2 after vaccination or after infection, and how they change over time.
The study will run in 13 European countries, Canada and the USA. In Europe, CAR-CF will run as a consortium. One site in each participating country will act as a sponsor for that specific country.
Please find more info in the CAR-CF infographic

Timeline
Investigators can enrol people with CF over a 12-month period. Participants will be followed up for 2 years.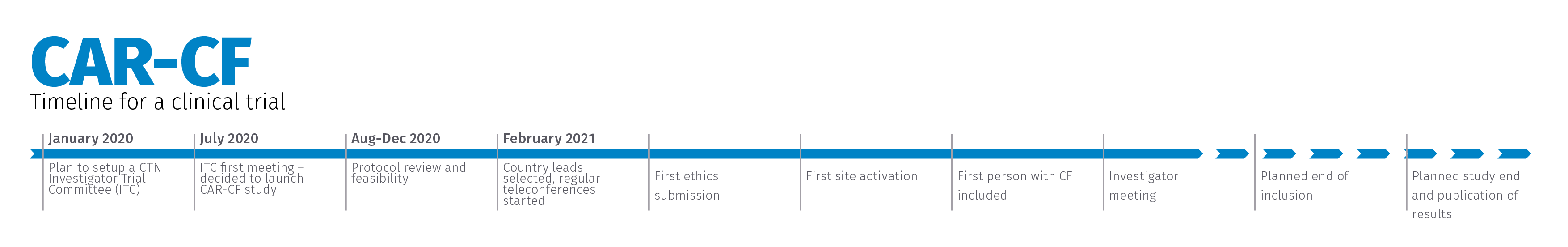
Study population
In Europe, CAR-CF will recruit people with CF from participating ECFS-CTN sites.
People with CF of any age, genotype, transplant status and disease severity can participate. People interested in the study can contact their CF team for more information.
CAR-CF is an observational study that collects blood samples and data only. Therefore CAR-CF participation doesn’t prevent participants from enrolling into other clinical trials testing new treatments. Participants already enrolled in a clinical trial can also enrol in this study.
Study design
CAR-CF will have up to five study visits over two years. The first study visit is called baseline or Day 0. The other study visits will be 6, 12, 18 and 24 months later. Participants have their study visits at the same time as their normal scheduled clinic visits, or when they are hospitalized.
At each study visit, researchers will collect a blood sample and will record clinical data from the participant’s health records.
If CAR-CF participants visit their clinic at any other time for blood tests, researchers will take an additional blood sample from the same blood-draw for CAR-CF.
Serum from blood samples will be shipped to a central laboratory at Queen’s University Belfast, Northern Ireland. Researchers at this lab will measure the level of SARS-CoV-2 antibodies in the blood samples.
Optional additional sample collection:
CAR-CF participants can choose to donate a second blood sample. Researchers will store plasma from this blood sample for future studies. Participants can also choose to allow researchers to store any serum leftover from antibody testing. The future studies will be about Covid-19 and CF, but the scientific questions have not yet been decided.
Status of CAR-CF in the participating countries
Please zoom in to see all participating sites in a specific country.
Acknowledgements
CAR-CF has received a research grant from the Cystic Fibrosis Foundation (CFF) to perform the study.
The French sites participating in the CAR-CF study will receive additional funding from the French patient organization - Vaincre la Mucoviscidose to conduct the trial.
The Dutch patient organization has contributed to support the Dutch site participating in CAR-CF.



We would like to thank the patient organisations for all the preparatory work in starting up the CAR-CF study.
A big thank you to the people with CF participating in the CAR-CF study. We would also like to thank all the country lead investigators, all the principal investigators, and research coordinators, in the CTN sites for their enthusiasm in successfully initiating the CAR-CF trial.
Contact
For information please contact the ECFS Clinical Trials Network ecfs-ctn@uzleuven.be
Principal Investigator: Dr Damian Downey
Co-Principal Investigator: Dr Silke van Koningsbruggen-Rietschel

