Which Patients should be Reported to the Registry?
The ECFS Patient Registry is a longitudinal study. It recruits all the prevalent cases on 1st January 2008 and all the subsequent incident cases.
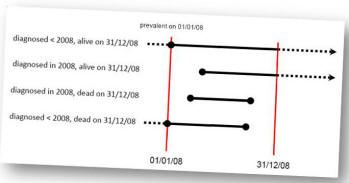
What are the inclusion criteria?
Only patients who fulfil the diagnostic criteria below should be included in the registry:
a. Two sweat tests >60 mmol/L chloride.
b. One sweat test >60 mmol/L chloride AND DNA Analysis/Genotyping - two identified disease causing CF mutations.
If the sweat value is less than or equal to 60 mmol/L, then at least 2 of these should be fulfilled:
a. DNA Analysis/Genotyping - two identified desease causing CF mutations.
b. Transepithelial (Nasal) Potential Difference - study consistent with a diagnosis of CF.
c. Clinical Presentation - typical features of CF.
Diagnosis reversal: if the patient's CF diagnosis reversed during the year, identify the reason from the options listed:
i. DNA Analysis - unable to identify two disease causing CF mutations.
ii. Transepithelial (Nasal) Potential Difference - study not consistent with a diagnosis of CF.
iii. Repeat normal sweat testing - confirm with clinical team.
In case of certain diagnosis reversal you should inform the registry in order to have this patient's records deleted from the registry.
References:
Gibson LE, Cook RE. A test for concentration of electrolytes in sweat in cystic fibrosis of the pancreas utilizing pilocarpine by iontophoresis. Pediatrics 1959;23:545-49
Farrell PM, Koscik RE. Sweat chloride concentrations in infants homozygous or heterozygous for F508del cystic fibrosis. Pediatrics 1996;97:524-8
De Boeck et al. Cystic fibrosis: terminology and diagnostic algorithms. Thorax 2006; 61(7):627-35.

