The European Cystic Fibrosis Society (ECFS) with its mission to improve survival and quality of life for people with CF strongly supports professional and educational activities that help improve the CF care in every European country. The ECFS patient registry output data show considerable discrepancy in clinical outcomes across Europe and to address this issue, the ECFS proposes the project of a long-term site-to-site partnership where one CF site guides, advises and helps another CF site to optimise the care for CF patients.



The Twinning Expansion Project
Announcement: New CF centres twinned to improve care across Europe
We are excited to announce the expansion of ECFS/CFE Twinning Project, which aims to build up friendships and partnerships between CF communities in different countries. It does so, first of all, by twinning one expert CF centre with a long-term outstanding history in CF care ("mentor site") to a "mentee site", a centre with the ambition to improve clinical outcomes through the guidance, advice and collaboration of a well-established CF centre.
Nine twins were established in 2020. After launching a new call to CF care providers in Europe last year, we had an impressive number of applications. The Twinning Expansion Project team is pleased to announce 21 new mentee CF Centres being mentored by 19 CF Centres.
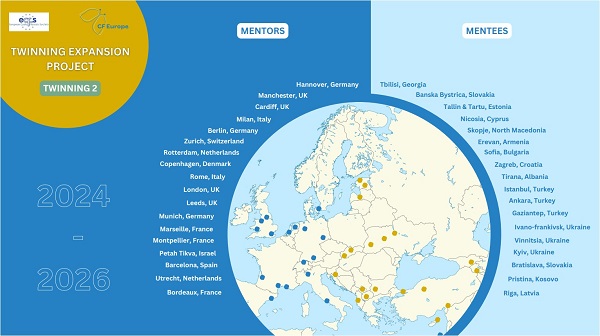
A full list of CF Centre twins:
| Mentee | Mentor |
| Tirana, Albania | Rome, Italy |
| Yerevan Muratsan, Armenia | Zurich, Switzerland |
| Yerevan Arabkir, Armenia | Zurich, Switzerland |
| Sofia, Bulgaria | Rotterdam, the Netherlands |
| Nicosia, Cyprus | Milan, Italy |
| Zagreb, Croatia | Copenhagen, Denmark |
| Tallin, Estonia | Cardiff, UK |
| Tartu, Estonia | Cardiff, UK |
| Tbilisi, Georgia | Hanover, Germany |
| Prishtina, Kosovo | Utrecht, the Netherlands |
| Riga, Latvia | Bordeaux, France |
| Skopje, North Macedonia | Berlin, Germany |
| Banska Bystrica, Slovakia | Manchester, UK |
| Bratislava, Slovakia | Barcelona, Spain |
| Ankara (Paediatric), Turkey | Leeds (Paediatric), UK |
| Ankara Adult, Turkey | Leeds (Adult), UK |
| Gaziantep, Turkey | Munich, Germany |
| Istanbul, Turkey | London (Kings), UK |
| Ivano-Frankivsk, Ukraine | Marseille, France |
| Kyiv, Ukraine | Petah Tikva, Israel |
| Vinnytsia, Ukraine | Montpellier, France |
These new twins join the nine twins that were established in 2020. The 2020 twins:
| Mentee | Mentor |
| Bucharest, Romania | Leuven, Belgium |
| Cluj-Napoca, Romania | Brussels, Belgium |
| Lviv, Ukraine | London (Brompton), UK |
| Odessa, Ukraine | Lyon, France |
| Zaporizhzhia, Ukraine | Jerusalem, Israel |
| Varna, Bulgaria | Southampton (Paediatric), UK |
| Athens, Greece | Southampton (Adult), UK |
| Thessaloniki, Greece | Southampton (Adult), UK |
| Kozle, North Macedonia | Cambridge (Adult), UK |
Working together to improve CF care throughout Europe
The Twinning Expansion Project (TEP) is a three-year (2024-2026) collaboration between CFE and ECFS, building on the ECFS Twinning Project (2020) which aimed to improve access to high quality, multidisciplinary CF care and optimal treatment.
The aim of the Twinning Expansion Project is to address discrepancies in clinical outcome for people with CF across Europe. Building long-term collaborative partnerships between mentors and mentee sites and addressing specific needs of the mentee sites, the Twinning Expansion Project will optimise patients' clinical outcomes.
Patient Organisations begin twinning!
A novel aspect of the Twinning Expansion Project is that it will also engage patient organisations, who play a pivotal role in identifying and addressing unmet needs (e.g. adult care, trained healthcare professionals), facilitating communication, and signalling possible mentee sites. Well-resourced patient organisations with advanced advocacy, digital expertise and capabilities will provide guidance to those patient organisations needing more support or training. As well as the twinned pairs building their partnership, the project also aims to support and facilitate networking between patient organisations and CF centres participating in the project.
Aims of the Twinning Expansion Project
1. Establish new CF centre mentor and mentee sites with the aim of facilitating collaborations and exchanges between the twinned pairs.
2. Identify unmet needs in CF care in the mentee sites and formulate feasible goals.
3. Grant access to ECFS learning resources to participating CF centres and translating these resources into relevant languages.
4. Facilitate knowledge sharing and networking opportunities between CF centres and patient organisations in different regions.
How twins will meet
It is expected of the new twinning pairs that the mentor site will organise at least one visit to the mentee site in 2024 (if geopolitical conditions allow it). The visit will include a CF physician and possibly a CF nurse and/or other CF team members. The aim of the visit is to establish a partnership, get an overview of the mentee site, discuss unmet needs and opportunities, and build on this knowledge to develop further twinning. The duration of the Twinning Expansion Project for new sites is one year. After one year, a summary report will be prepared and submitted jointly by both sites and will serve as the basis for the continuation of the funding.
For more information contact: